PIPELINE
TU2218
(A Novel, Oral Immuno-Oncology Therapy)
Unlike conventional anticancer drugs that include chemotherapy and targeted therapies, immuno-oncology drugs, also called immune checkpoint inhibitors, stimulate the immune system of cancer patients and induce immune cells to attack cancer cells. Immuno-oncology drugs are becoming the standard for anti-cancer treatment because they have lower side effects and substantially improved survival rates compared to first-generation chemotherapy and second-generation targeted cancer drugs. However, immuno-oncology drugs are limited in their low response rate, which is effective only in about 15% to 30% of patients with cancer depending on the indication. Accordingly, the development of combination therapy is being actively conducted to increase the response rate of immuno-oncology drugs and maximize the therapeutic effect.
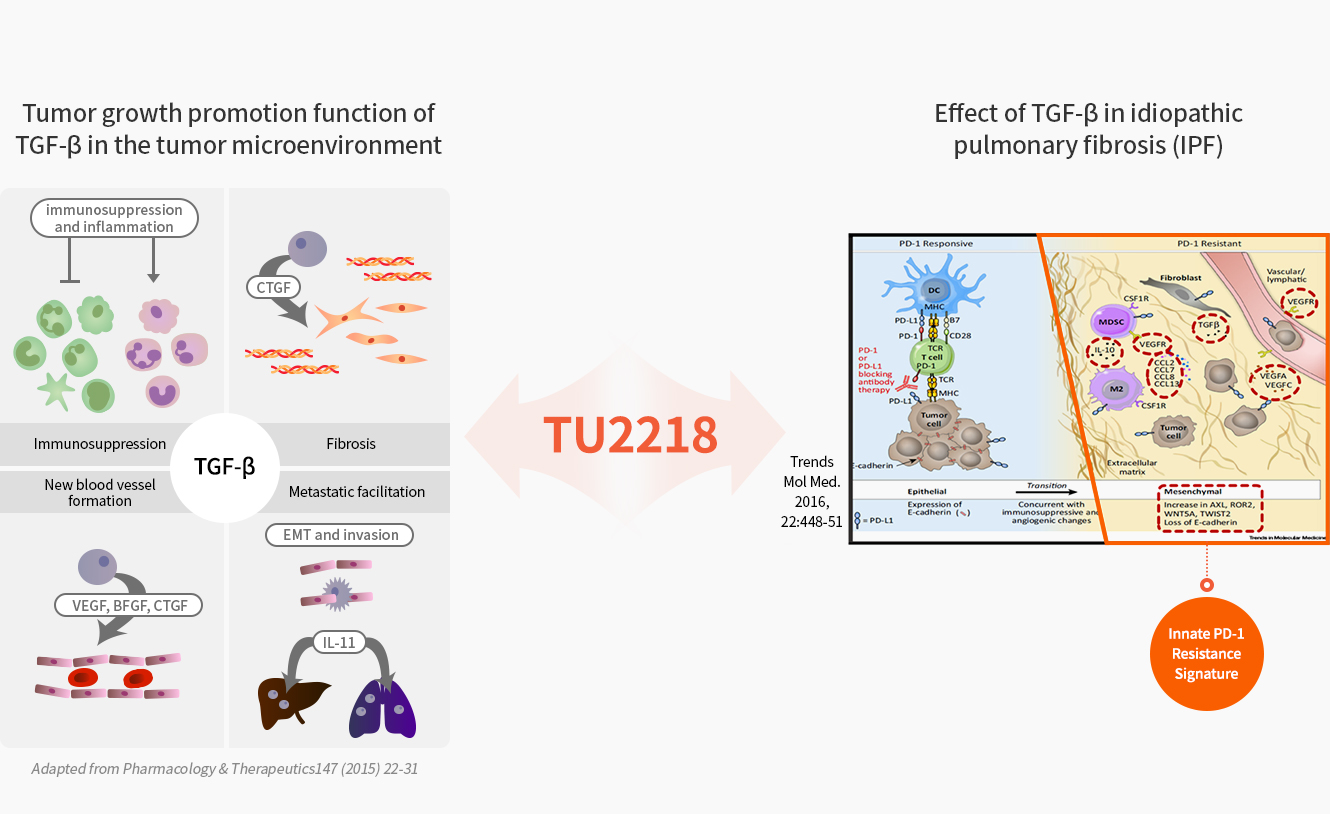
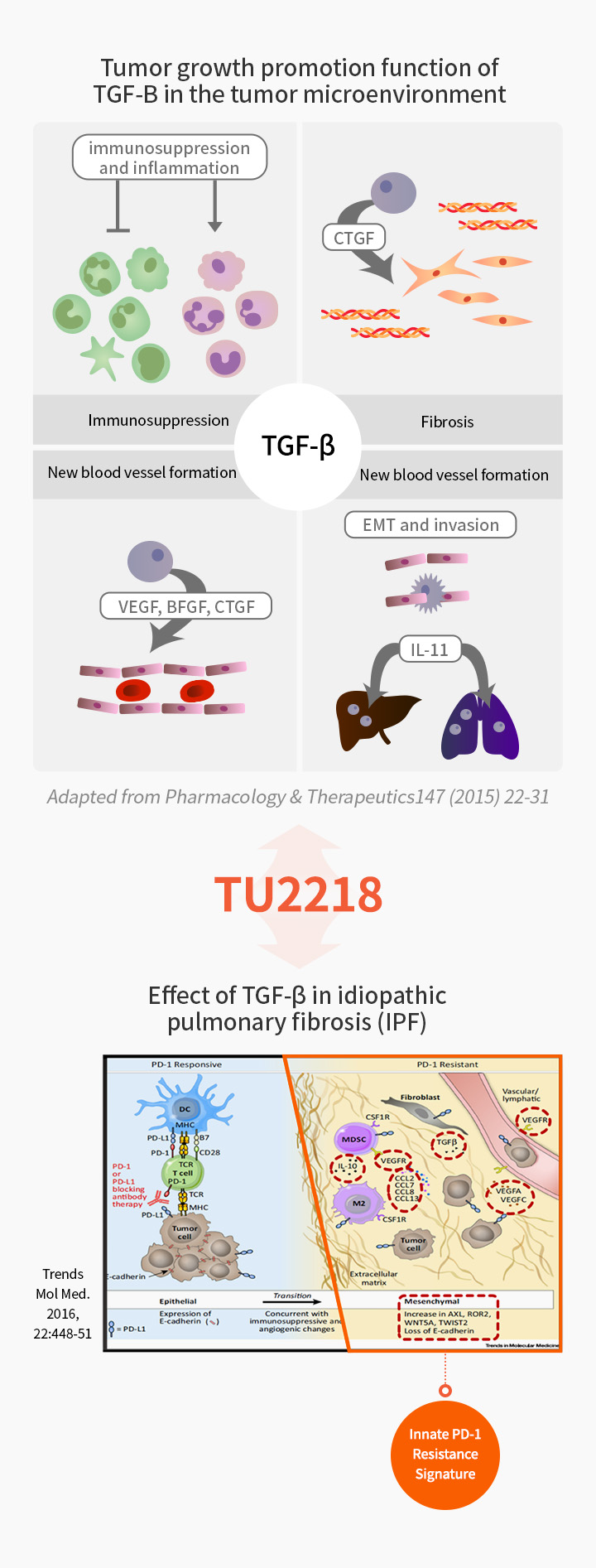
Overexpressed TGF-β in the tumor microenvironment is reported as a major factor that hinders the efficacy of anti-PD1 drugs. VEGF is a proven target in terms of synergy with immuno-oncology drugs and is the most preferred mechanism for clinical trials in combination with immuno-oncology drugs since 2021.
TU2218 is a potentially ‘first-in-class’ immuno-oncology drug that simultaneously inhibits TGF-β and VEGF. It is expected to resolve unmet clinical needs when administered in combination with immune checkpoint inhibitors such as PD-1 inhibitors and CTLA-4 inhibitors.
We are currently conducting a clinical trial of TU2218 in combination with the PD-1 inhibitor ‘Keytruda (pembrolizumab)’ under the approvals by FDA and MFDS.
