PIPELINE
TU2670
(A Once-Daily, Oral, Nonpeptide GnRH Antagonist for the Treatment of Endometriosis/Uterine Fibroids)
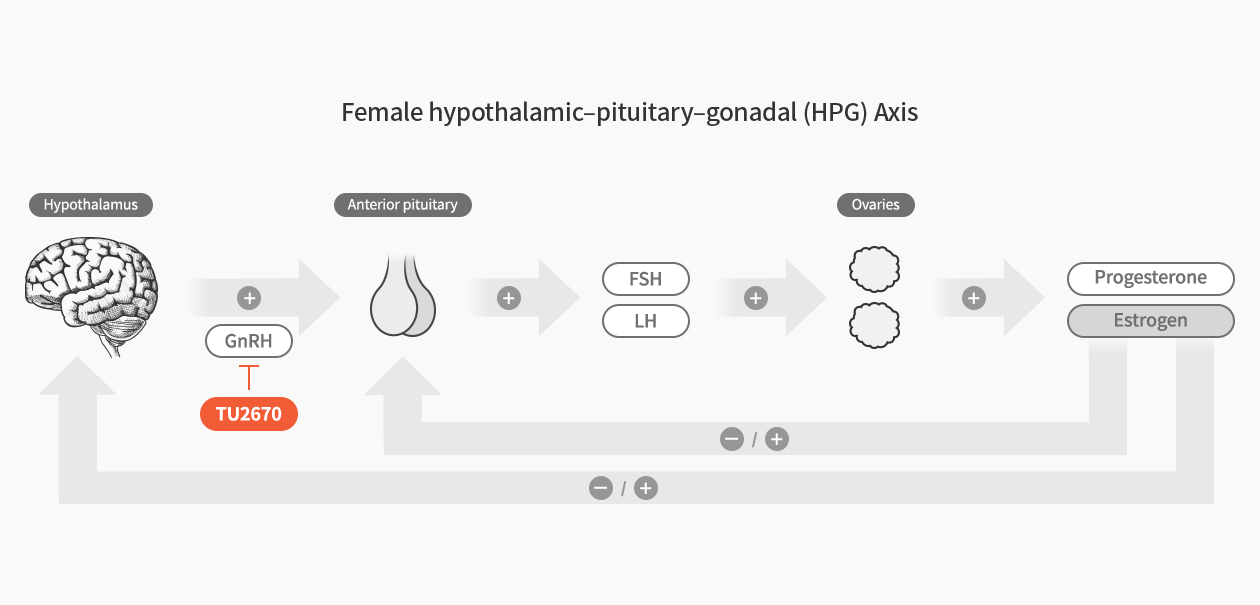
Endometriosis is a disorder in which the endometrial tissue that line the inside of the uterus grows out the uterus and is known to cause severe pelvic pain, menstrual pain, lower abdominal pain, and infertility which occurs in about 10% of women in childbearing age.
Uterine fibroids is the most common benign tumor that occurs in the uterus, and is a condition that accompanies symptoms such as pain, excessive menstruation, dysfunctional uterine bleeding, and urinary incontinence which is a common disease that occurs in about 30% of women in childbearing age. Both diseases are chronic diseases, and patients are demanding safer treatment with fewer side effects for long-term use due to serious side effects such as bone loss of existing treatments.
TU2670 is an oral GnRH antagonist that can bind to pituitary receptors to suppress estradiol hormone. The existing treatment GnRH agonists, lowers patient comfort levels as it’s in an injection form and inhibits sex hormones to the level of menopause, resulting in serious side effects such as bone loss. TU2670 is an oral GnRH antagonist that only suppresses hormone levels to the level of efficacy, improving both side effects and ease of administration by oral. In addition, being a sex hormone control mechanism, it allows for indication expansion to precocious puberty, test-tube baby, infertility treatment, etc.
Currently, we are conducting a Phase 2a clinical trial of TU2670 in endometriosis in 5 European countries. In 2019, we signed an exclusive licensing agreement with Deawon Pharma for the development and commercialization of TU2670 in South Korea, and Deawon is currently conducting a Phase 2 clinical trial in South Korea in uterine fibroids. In 2022, we entered into a $170M licensing agreement with Hansoh Pharma to develop and commercialize TU2670 in Greater China. Currently, Hansoh is carrying out clinical development of TU2670 in China.
